Stryker’s Sustainability Solutions (SSS) is committed to sustainable healthcare. Since the Ascent acquisition in 2009, we have been changing the way healthcare thinks about “single-use” devices. A successful reprocessing program needs regular, disciplined collection pick-ups, fast product turnaround times, frequent staff in-services, clinical education, physician engagement/case support, routine savings reporting, highly visible and responsive account representation, and quarterly business review meetings. Our OnTrack team of sales reps, service associates, corporate account managers, analysts, internal support teams, and their collective leadership is best positioned to deliver the best reprocessing results.
SSS understands consistent medical device performance is critical in gaining clinician confidence. We implemented the same quality system utilized at other Stryker divisions and have continued to push the boundaries of simulated-use performance testing and multi-point inspections. Today, over 3,000 hospitals in the US and abroad trust Stryker to reprocess their devices. We’ve delivered over time, and we believe consistent device quality and great customer service are the most important ingredients in a successful reprocessing program.
Featured products
Learn more about the single-use devices that SSS reprocesses.
The circular economy is an economic system based on the reuse and regeneration of materials or products, especially as a means of continuing production in a sustainable or environmentally friendly way. Hospitals across the nation are focued on reducing their environmental footprint. As a reprocessor, Stryker's Sustainability Solutions collects devices and then inspects, cleans, function tests, sterilizes and packages devices for safe reuse following strict FDA guidelines.
SSS maximizes savings and furthers the circular economy by extending the useful life of single-use medical devices (SUDs).
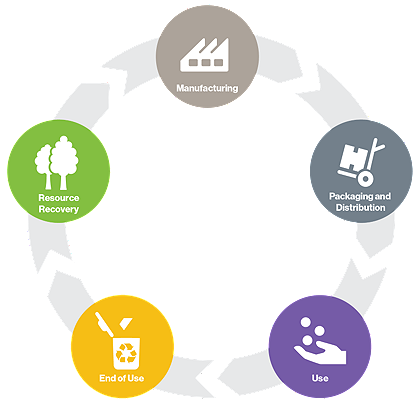
Redesigned for sustainability
As a next evolution of reprocessing, we launched our Redesigned for Sustainability platform in 2020. This takes a lifecycle approach to bringing new products to market and incorporates sustainable design into sourcing, packaging, component design, materials and chemicals, as well as manufacturing and waste solutions.
Redesigned for Sustainability was created to align with our customers sustainability initiatives like Environmentally Preferable Purchasing, harmful chemical reduction and waste reduction goals.


Products for the planet
To further our sustainability efforts, and drive stronger clinical staff engagement, we developed our Products for the Planet program which provides hospitals the opportunity to contribute to the restoration of National Forests by achieving annual collection goals for single-use devices.
Because of the efforts of our customers, we’ve been able to plant over more than 220,000 trees that have helped restore National Forests in Oregon, Arizona, West Virginia and Georgia.
Additionally, we have partnered with Tree Canada to expand our environmental reach.
There isn’t a one size fits all solution when it comes to sustainability, but we are proud of the progress we have made thus far and are excited to continually improve, evolve and learn with our customers.
Environmental Excellence Awards
At SSS, we know our choices impact the health of the planet and the people within it. This is why we are so proud of the 3,898 hospitals that received an Environmental Excellence Award for their 2023 efforts. This award takes into account collections growth, buyback., and waste diverted at each facilitiy SSS partners with. We look forward recognizing even more winners in 2025!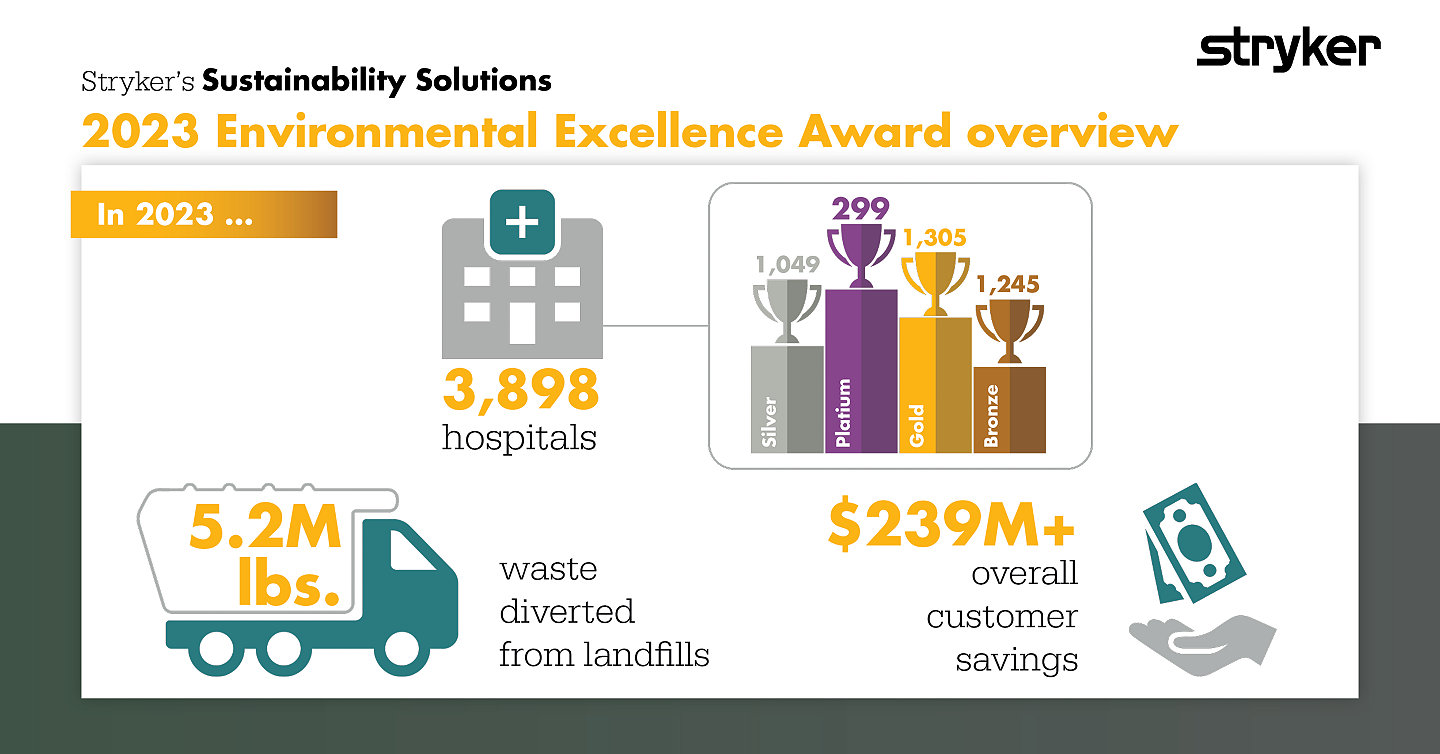

Stryker’s Sustainability Solutions has been recognized as one of the Top Companies to Work for in Arizona by Best Companies AZ!
This incredible achievement is a testament to our unwavering commitment to fostering a positive, innovative, and supportive work environment for all our team members. Together with our customers, we're making a difference in the healthcare industry by providing sustainable solutions and furthering the circular economy.
Learn more about what Stryker Sustainability Solutions can do for you
Connect with Stryker
SSS is now on LinkedIn, Instagram and Twitter
CIS11249

